Introduction
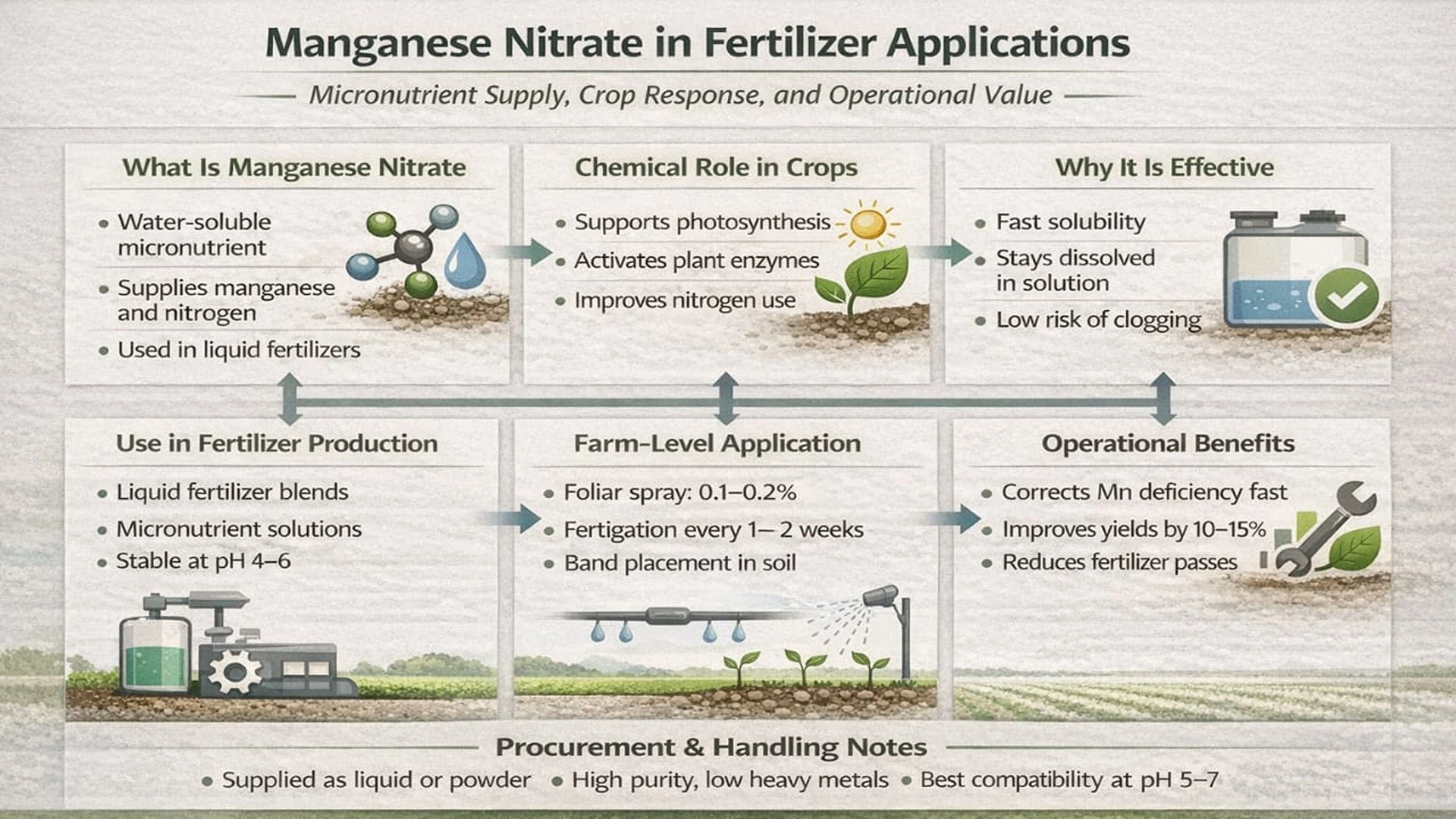
Manganese nitrate is a water-soluble fertilizer input used to supply manganese and nitrogen to crops. It is widely applied where soils lack available manganese, especially in high-pH or calcareous conditions. Fertilizer producers and buyers value manganese nitrate for its fast solubility, easy mixing, and good performance in liquid fertilizers, fertigation, and foliar feeding systems.
Chemical Role of Manganese Nitrate in Fertilizers
Manganese nitrate (Mn(NO₃)₂) works mainly as a micronutrient source. It provides manganese in a form that plants can absorb quickly. Manganese is needed for photosynthesis, enzyme activity, and nitrogen use inside the plant. Without enough manganese, crops show yellow leaves and weak growth.
The nitrate part adds nitrogen that plants can take up fast. This helps early growth and supports protein formation. Unlike less soluble manganese products, manganese nitrate stays dissolved in water. This reduces the risk of clogging or settling in tanks and pipes.
Because of its simple salt structure, manganese nitrate dissolves easily and mixes well with many fertilizer solutions. This makes it suitable for liquid fertilizers and tank mixes without the need for complex chelating agents.
Role in Fertilizer Production
Manganese nitrate is added during the production of liquid fertilizers and micronutrient blends. It is commonly made by reacting manganese carbonate with nitric acid. The result is a clear solution that can reach about 50 percent concentration.
In fertilizer plants, manganese nitrate is added after pH adjustment to avoid breakdown of the solution. A pH range of about 4 to 6 helps keep it stable. It blends well with calcium nitrate, ammonium nitrate, and urea solutions used in water-soluble fertilizers.
For dry fertilizer use, spray-drying can produce a powder form. Direct granulation is less common because manganese nitrate absorbs moisture easily. Quality checks focus on manganese content and low impurity levels to meet fertilizer standards.
Application at Farm Level
Manganese nitrate is used at different crop stages, depending on the deficiency level. Foliar spraying is common because it delivers manganese directly to leaves. Typical spray rates are 0.1 to 0.2 percent solution, applied during early growth or before flowering.
In fertigation systems, manganese nitrate is injected into drip or sprinkler lines. This allows even distribution in soils where manganese is tied up by high pH. Applications are often repeated every one to two weeks during active growth.
Soil application is done before planting, usually mixed with nitrogen fertilizers. Band placement reduces the total amount needed and improves efficiency compared with full broadcasting.
Differences Across Crop Sectors
Use of manganese nitrate varies by crop type. Row crops such as wheat, rice, and vegetables often rely on foliar sprays to correct fast deficiencies. This approach avoids nutrient loss from leaching.
Tree crops and fruit orchards use lower spray rates focused on young leaves during early growth stages. Horticulture and greenhouse crops prefer fertigation, where precise dosing improves uniformity and crop quality.
In precision farming systems, manganese nitrate is often used together with controlled-release nitrogen fertilizers. This helps reduce nutrient loss and improves overall efficiency. Berry and vine crops may receive several smaller applications, while broad-acre crops limit treatments to control costs.
Operational Value for Fertilizer Users
Manganese nitrate improves fertilizer efficiency by helping plants use nitrogen better. It supports enzyme systems that turn nitrate into plant protein. This can improve nitrogen use efficiency by up to 30 percent in deficient soils.
Its high solubility allows lower application rates compared with manganese sulfate. Better crop response can increase yields by 10 to 15 percent where manganese deficiency is present.
From an operational view, manganese nitrate mixes well with other fertilizers and crop protection products. This reduces the number of field passes needed, saving labor and fuel. Liquid forms also lower transport and handling costs in large fertilizer programs.
High-purity grades meet strict limits on heavy metals. This helps fertilizer producers meet regulatory rules in major markets. Compared with sulfate forms, manganese nitrate has a lower risk of increasing soil acidity.
Procurement and Handling Considerations
Fertilizer buyers usually source manganese nitrate as a 24 to 50 percent manganese product. It is supplied in bags, drums, or bulk containers. Buyers check safety data sheets for handling and storage rules, as the product is corrosive and should be kept away from organic materials.
Prices depend on nitric acid and raw material costs. Suppliers using carbonate-based production routes offer stable quality and low impurity levels. Compatibility tests are done before large-scale blending, with best results at pH 5 to 7.
Conclusion
Manganese nitrate is an effective and flexible fertilizer input for correcting manganese deficiency and improving nitrogen performance. Its high solubility, fast plant response, and good mixing behavior make it well suited for liquid fertilizers, fertigation, and foliar feeding. For fertilizer producers and growers, it offers strong efficiency gains, lower application rates, and reliable crop response. As demand grows for precise and efficient nutrient use, manganese nitrate will continue to play an important role in modern fertilizer programs.
Leave a Comment